Los Angeles, CA, MAY 8, 2024 – TETROUS, INC., a leading regenerative medicine company today announced that it has completed the first surgical cases using EnFix TAC™, its latest product that expands its line of EnFix™ implants to cover every surgical technique for rotator cuff repair.
The EnFix product line is specifically designed to address Enthesis Failure Syndrome.
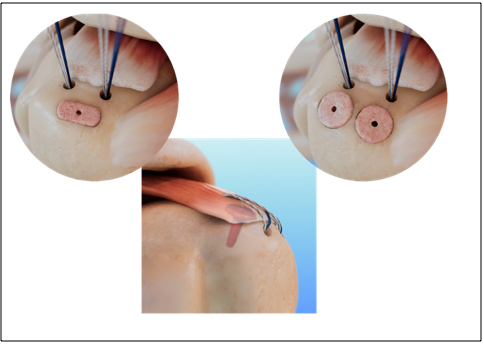
The EnFix technology utilizes proprietary demineralized cortical bone fibers to yield an osteoinductive and osteoconductive implant that promotes optimal biologic healing at the bone-to-tendon interface, resulting in reformation of the enthesis. With strong pre-clinical data and promising early clinical results showing healing at the bone-to-tendon interface, there is growing evidence supporting the applicability of the EnFix technology to address the unmet need for technology that can improve on the weak repair at the enthesis that is driving the high surgical failure rates in rotator cuff repairs.
The EnFix TAC® is the second offering in the EnFix family of implants and is available in two configurations, TAC-O and TAC-T, to accommodate the varied availability of bony real estate based on the size of the rotator cuff tear. While the EnFix RC® is designed to be used with suture anchors, the EnFix TAC® options are specifically designed to deliver the same enthesis healing properties in a graft that is intended for use independent of a suture anchor or with all-suture anchors.
Nikhil Verma, MD, Professor and Director, Sports Medicine and Shoulder, Midwest Orthopedics at Rush, Head Team Physician, Chicago White Sox, who performed the first surgeries with the EnFix TAC said, “When it comes to surgical outcomes in rotator cuff repair, enthesis healing is one of the most critical factors. The EnFix implants address this crucial need with minimal disruption to the surgical procedure”. He continued, “I believe these products will have a transformative impact on surgical protocols in rotator cuff repair”.
Since June 2023, in the initial commercial release of the EnFix implants, more than 400 grafts have been implanted in over 200 surgical cases in the US and Australia.
###
About Tetrous, Inc.
Founded in 2019, Tetrous, Inc. utilizes next generation advanced technologies for enthesis repair in sports medicine applications. The first offerings in the EnFix family of products are EnFix RC and EnFix TAC for rotator cuff repair. Tetrous was conceived from technology developed for spine surgery. The Company is employing and expanding the technology for novel applications in sports medicine. Its core technology has been used in over 150,000 implants in spine applications. Tetrous enjoys significant IP protection for its EnFix family of products with multiple issued patents and, additionally, has an exclusive license to the demineralized bone fiber technology used in its products for sports medicine applications from TheraCell, an ISTO Biologics Company.
EnFix®, EnFix RC™, EnFix TAC™, and It’s All About the Enthesis™ are trademarks of Tetrous, Inc.
FormLok™ is a trademark of TheraCell, Inc., an Isto Biologics company.
For more information visit www.tetrous.com and follow us on LinkedIn (www.linkedin.com/company/tetrous).

