LOS ANGELES, July 11, 2023 – Tetrous Inc., a leading regenerative medicine company today announced that it has completed the first four surgical cases using EnFix RC®, a procedure-specific implant for rotator cuff repair that is specifically designed to address Enthesis Failure Syndrome. Dr. Nikhil Verma, MD. Professor and Director, Division of Sports Medicine, Fellowship Director, Department of Orthopedics, Rush University Medical Center, Team Physician Chicago White Sox and Chicago Bulls, who performed the first four surgeries with the EnFix RC® said: “I was thrilled to be the first surgeon to deploy the EnFix RC®. I used one EnFix RC® in each case to augment the medial suture anchor fixation. Initially I was impressed with the concept of flipping the script compared to most of the products today for surgical augmentation. I also was very pleased with how the implant and instrument design was optimized, simplifying the delivery of the product into the interface between the tendon and the bone. The procedure is minimally disruptive to the way rotator cuff surgeries are typically performed. I plan to use the EnFix RC® routinely moving forward.”
In the United States alone, nearly 500,000 rotator cuff repairs are performed annually and 20% to 70% of these repairs fail structurally. Inadequate tendon-to-bone ingrowth results in incomplete healing, gap formation and a high risk of re-tear. Tendon reattachment is a crucial clinical need, especially in larger tears because failure rates increase linearly with tear size. While most augmentation products have been designed as “overlays” to reinforce the tendon, EnFix RC changes the paradigm by enhancing healing at the enthesis where failure often occurs. This enhanced biologic repair at the interface between tendon and bone is a significant advance.
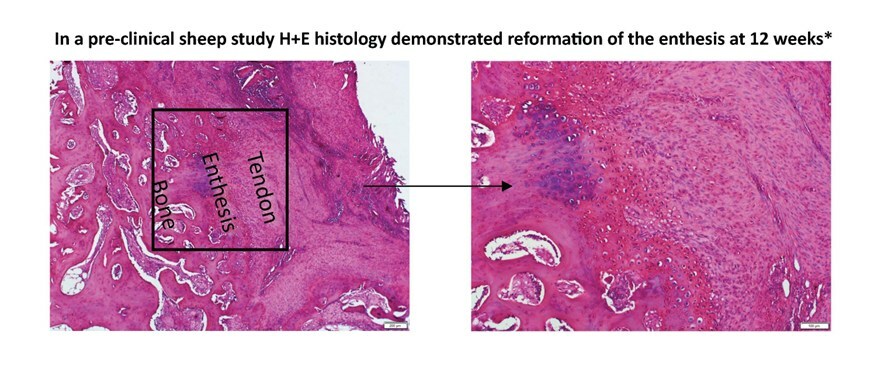
John Bojanowski, Tetrous’ Head of Sales and Marketing said: “We are proud to offer a highly differentiated clinical solution. EnFix RC specifically promotes the remodeling of tissue approximating that of the natural tendon-bone interface, known as the enthesis. The enthesis is where failures often occur in sports medicine repairs such as Rotator Cuff, ACL and Achilles. Tendon reattachment to the bone is a crucial clinical need, especially in larger tears, since failure rates increase linearly with tear size.”
Tetrous CEO, Bradley Patt, PhD added: “Our technology has a long clinical legacy in Orthopaedics and has been extensively studied in tendon and bone healing demonstrating both osteoconductivity and osteoinductivity. The demineralized bone fibers in EnFix RC promote optimal biologic performance while easily integrating into current surgical techniques. It is all about the enthesis”.
The EnFix RC® rotator cuff implant is made from proprietary demineralized cortical bone fibers. Using Bone Textile™ technology to produce the implant yields long and strong bone fibers that promote cell wicking, cell adhesion and cell proliferation, while our FormLok™ technology imparts shape retention to the device, even when immersed in liquid, as is regularly required for use in arthroscopic surgery.
###
About Tetrous, Inc.
Founded in 2019, Tetrous, Inc. utilizes next generation advanced technologies for enthesis repair in sports medicine applications. The first offerings in the EnFix family of products are EnFix RC and EnFix TAC for rotator cuff repair. Tetrous was conceived from technology developed for spine surgery. The Company is employing and expanding the technology for novel applications in sports medicine. Its core technology has been used in over 150,000 implants in spine applications. Tetrous enjoys significant IP protection for its EnFix family of products with multiple issued patents and, additionally, has an exclusive license to the demineralized bone fiber technology used in its products for sports medicine applications from TheraCell, an ISTO Biologics Company.
EnFix®, EnFix RC™, EnFix TAC™, and It’s All About the Enthesis™ are trademarks of Tetrous, Inc.
FormLok™ is a trademark of TheraCell, Inc., an Isto Biologics company.
For more information visit www.tetrous.com and follow us on LinkedIn (www.linkedin.com/company/tetrous).