The EnFix products are allogenic tissue products that conform to the Food and Drug Administration’s (FDA) regulations governing human and cellular tissue-based products (HCT/P) according to 21 CFR Part 1271 and Section 361 of the PHS Act. The processes used to manufacture EnFix products were designed to cause minimal changes to the allograft tissue and to maintain the osteoinductive potential. The products are 100% cortical bone and contain no additives or excipients.
While DBM is a potent biomaterial, in its most-used form as a powder it has poor handling characteristics and lacks osteoconductivity. Excipients used to make DBM powder easier to handle can contain up to 70% extraneous binding materials that have no beneficial value as biomaterials and do not address the lack of osteoconductivity. Early demineralized bone fiber technologies improved osteoconductivity as superior putties but did not offer a means to yield shaped allograft. Tetrous utilizes a second-generation fiber technology that overcomes many of the limitations of earlier manufacturing methodologies and provides a means of producing procedure-specific shaped products while also yielding highly osteoinductive and osteoconductive properties.
EnFix White Paper
For a comprehensive review of the science behind the EnFix family of allograft implants, please reference the white paper below, “EnFix Implants for Enthesis Repair”.
FORMLOK™ TECHNOLOGY
Production of implants with intricate geometries required the development of a proprietary patented water assisted injection molding (WAIM) process that permits fibers to be suspended as a slurry and injected into shaped molds. A further process, designated FormLok, allows the shape to be retained to control implant integrity, even in a wet environment, as is often required in arthroscopic surgery.
The figures below show timelapse images of EnFix with and without FormLok treatment. The nontreated sample on the left in each image rapidly loses its shape, while the FormLok treated device on the right retains its shape at 15 minutes, and beyond.
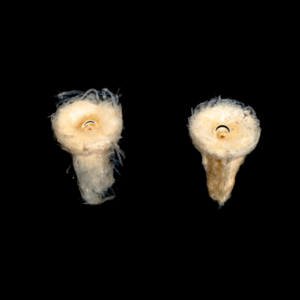
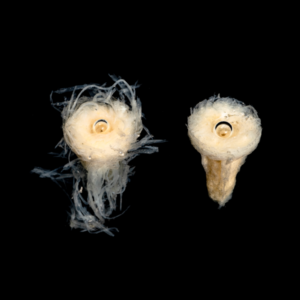
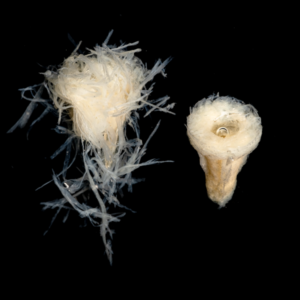
NANOTOPOGRAPHY
The topography of a surface can influence cellular response. Traditional demineralization techniques apply acid treatment after the fiber is formed, etching the critical nanotopography (surface features) away. Tetrous’ proprietary process demineralizes bone struts first and then cuts the fibers along the bone’s long axis, creating long and strong fibers and maintaining the collagen structure of bone, while also preserving the inductive proteins (the BMPs).
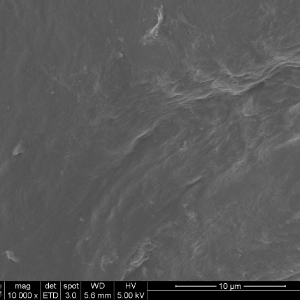
The DBF fiber nanotopography of the Tetrous fibers is shown in the images on the left below. In contrast, the images on the right show a conventional DBM particle and demonstrate how the acid treatment smooths the surface and destroys the beneficial topography.


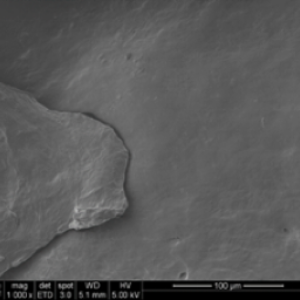
PRE-CLINICAL SHEEP STUDIES
Rotator Cuff Repair
In the United States alone, nearly 500,000 rotator cuff repairs are performed annually and 20% to 70% of these repairs fail structurally. Inadequate tendon-to-bone ingrowth results in incomplete healing, gap formation and a higher risk of re-tear. Tendon reattachment is a crucial clinical need, especially in larger tears because failure rates increase linearly with tear size. Most augmentation products have been designed as “overlays” to reinforce the tendon. EnFix RC® and EnFix TAC® change the paradigm by enhancing healing at the enthesis where failure often occurs. This enhanced biologic repair at the interface from the bone to the tendon is a significant advance.
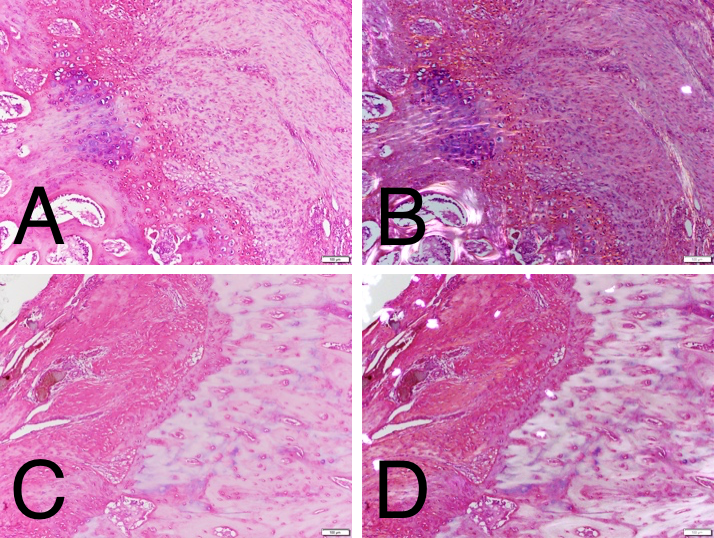
In an ovine infraspinatus tendon model of enthesis repair, a DBF sheet was placed at the interface between tendon and bone.
H&E histology at 12 weeks under normal light for the DBF treated (A) and control (C) revealed an active interface with some residual DBF and enthesis reformation. Polarized light confirmed Sharpey’s fibers in the DBF treated group (B) that were not present in the controls (D).
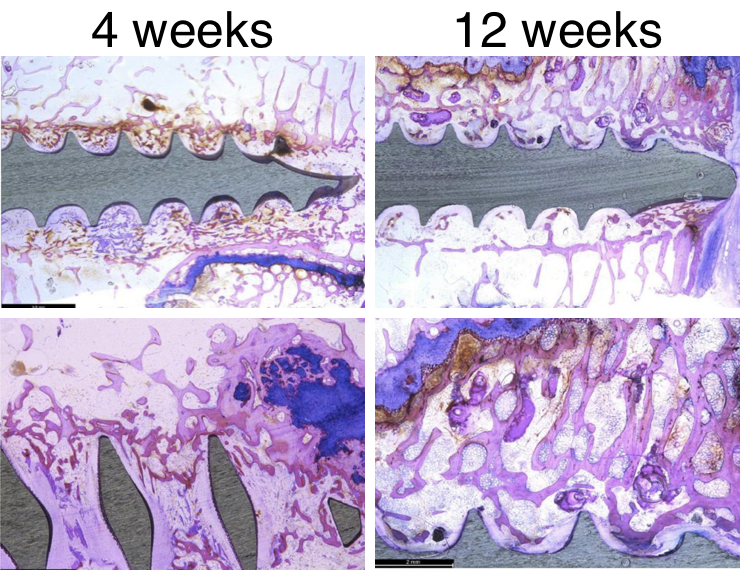
In a separate study, 6.5mm diameter bone screws were placed into fiber sleeves mimicking the peg portion of the EnFix RC® implant and placed into distal femoral condyles of skeletally mature sheep. The study showed that the DBF fibers facilitated new bone formation around the screw.
New bone formation with some residual DBF can be seen at four weeks (left), while at 12 weeks (right), all of the DBF has remodeled into new woven bone in the areas between the screw threads.
RABBIT INFRASPINATUS REPAIR
In a rabbit infraspinatus model, the tendon was detached and reattached using suture fixation through two bone tunnels. Demineralized bone fiber was placed between the tendon and the bone in the treated group. In the control group the tendon was reattached without the DBF sheet. Sutures were cut prior to mechanical testing at each time interval.
Tensile testing of the repaired rotator cuffs was performed and DBF and non DBF repairs compared at 6 and 12 week timepoints. The cuff repairs in the DBF treated group required greater force to detach the tendon from the bone than those in the control group and the control was not statistically higher at 12 weeks vs. 6 weeks.
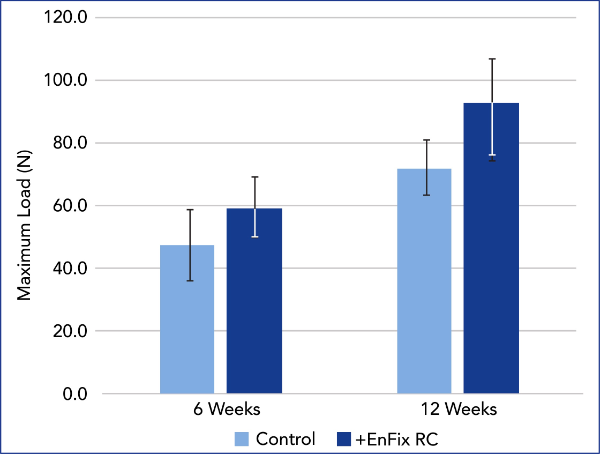
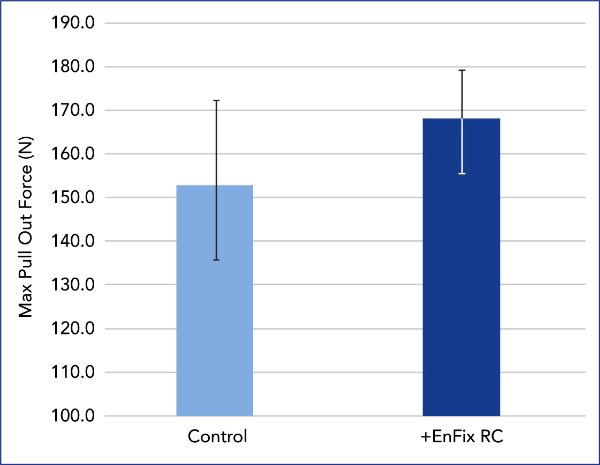
SUTURE ANCHOR PULL OUT TESTING
Evaluation of suture anchor fixation in the EnFix RC® implant was performed in Sawbones Foam (1522-09; 10 pfc), a bone analog specified in ASTM standards for pull out testing. In the treated group, an EnFix RC® implant (4.5mm) was inserted in the usual manner and a 5.5mm PEEK suture anchor was gently tapped into the device. The control group used a suture anchor alone. Pull out testing was performed at 20 mm/min, with maximum load recorded.
The EnFix RC® is shown to provide a modest improvement in fixation.
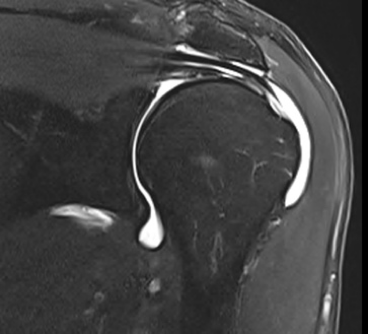
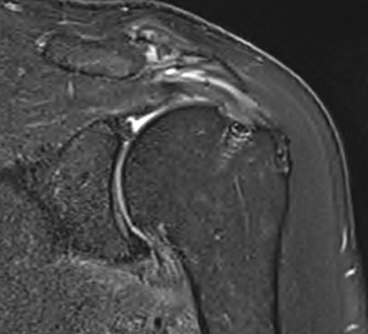
MRI CLINICAL ASSESSMENT
MRI assessment of repairs using the EnFix RC® and EnFix TAC® is being conducted on patients at 3 months and 6 months post op. Pre-Op and 6 Month Post-Op MRIs are provided below as examples. The post-op MRI demonstrates excellent healing of the supraspinatus tendon repair at the greater tuberosity footprint with clear tendon-to-bone ingrowth. There is high quality coverage of the greater tuberosity footprint by the repaired tendon similar in morphology to what a native tendon would look like. The only evidence that a tear has been repaired, other than the bone anchors themselves, is the mild intermediate T2 signal in the tendon substance. Note that there is little to no T2 signal between the tendon and the adjacent footplate bone, which suggests a robust tendon-bone enthesis. The EnFix TAC® shows successful integration into the surrounding bone, with nearly no visible evidence of the EnFix TAC® such as marrow edema, cystic change or adverse localized soft tissue reaction. The suture anchor is well seated in the bone and is easily visible with no artifact obscuring the tendon insertion.